
agpyo [at] stanford [dot] edu
About Me
I am a postdoctoral researcher (Stanford Science Fellow) at Stanford University, working with Ben Good at the intersection of physics and immunology. My research focuses on developing mathematical models to uncover the fundamental biological principles that govern immune system function.
I received my Ph.D. in Physics from Princeton University, where I worked with Ned S. Wingreen, supported by the NSERC Postgraduate Scholarship (PGS D). My research combines theoretical physics and biology to explore how macroscopic properties emerge from microscopic interactions. During my Ph.D., I investigated the physics of biomolecular condensates and developed theoretical models of the adaptive immune system, often in close collaboration with both theorists and experimentalists.
Before graduate school, I received a B.Sc. in Engineering Physics from the University of Alberta, where I worked with Michael T. Woodside on single-molecule biophysics, studying barrier-crossing dynamics in DNA hairpin folding.
Education
- Ph.D. in Physics, Princeton University, 2025
- M.A. in Physics, Princeton University, 2022
- B.Sc. in Engineering Physics, University of Alberta, 2020
Research Interests
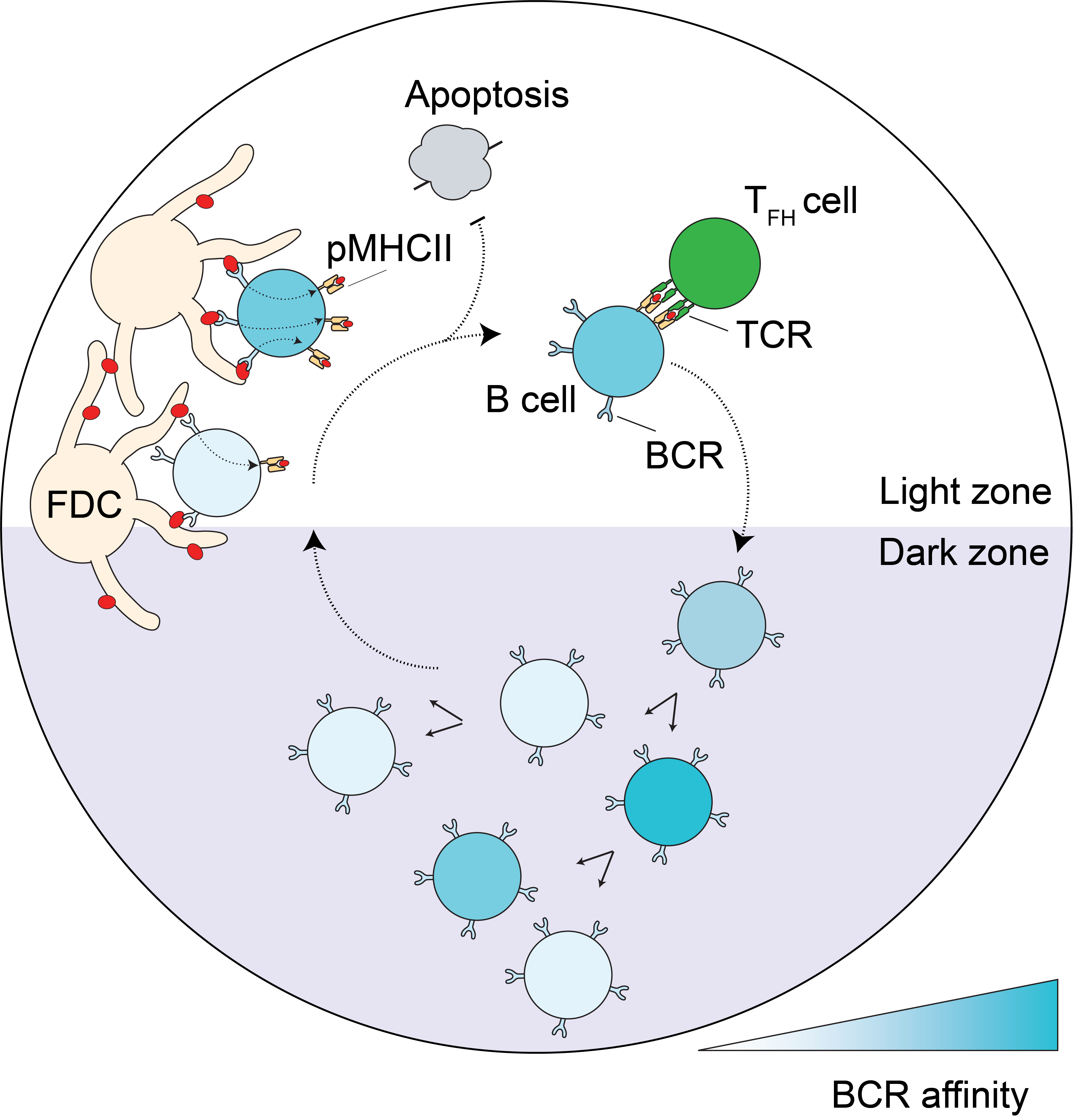
Plasma cells that secrete high-affinity antibodies, along with memory B cells, play a central role in humoral immunity. These specialized cells arise through the process of B cell affinity maturation—a dynamic evolutionary process in which B cells undergo iterative cycles of selection, proliferation, and somatic hypermutation to improve antibody affinity for a target antigen.
I am broadly interested in understanding how this complex process is regulated: What principles govern the efficiency and fidelity of affinity maturation? How do cellular and molecular mechanisms shape the evolutionary trajectory of lymphocytes? And how might these processes be tuned or perturbed in vaccination, infection, or disease?
- Regulated somatic hypermutation enhances antibody affinity maturation. Nature (2025)
Merkenschlager*, J., Pyo*, A. G. T., Silva Santos, G. S., Schaefer-Babajew, D., Cipolla, M., Hartweger, H., Gitlin, A. D., Wingreen, N. S., & Nussenzweig, M. C. - Evolutionary benefits of fitness-dependent mutation rates. PRL (2025)
Pyo, A. G. T., Yu, Q., Merkenschlager, J., & Wingreen, N. S.
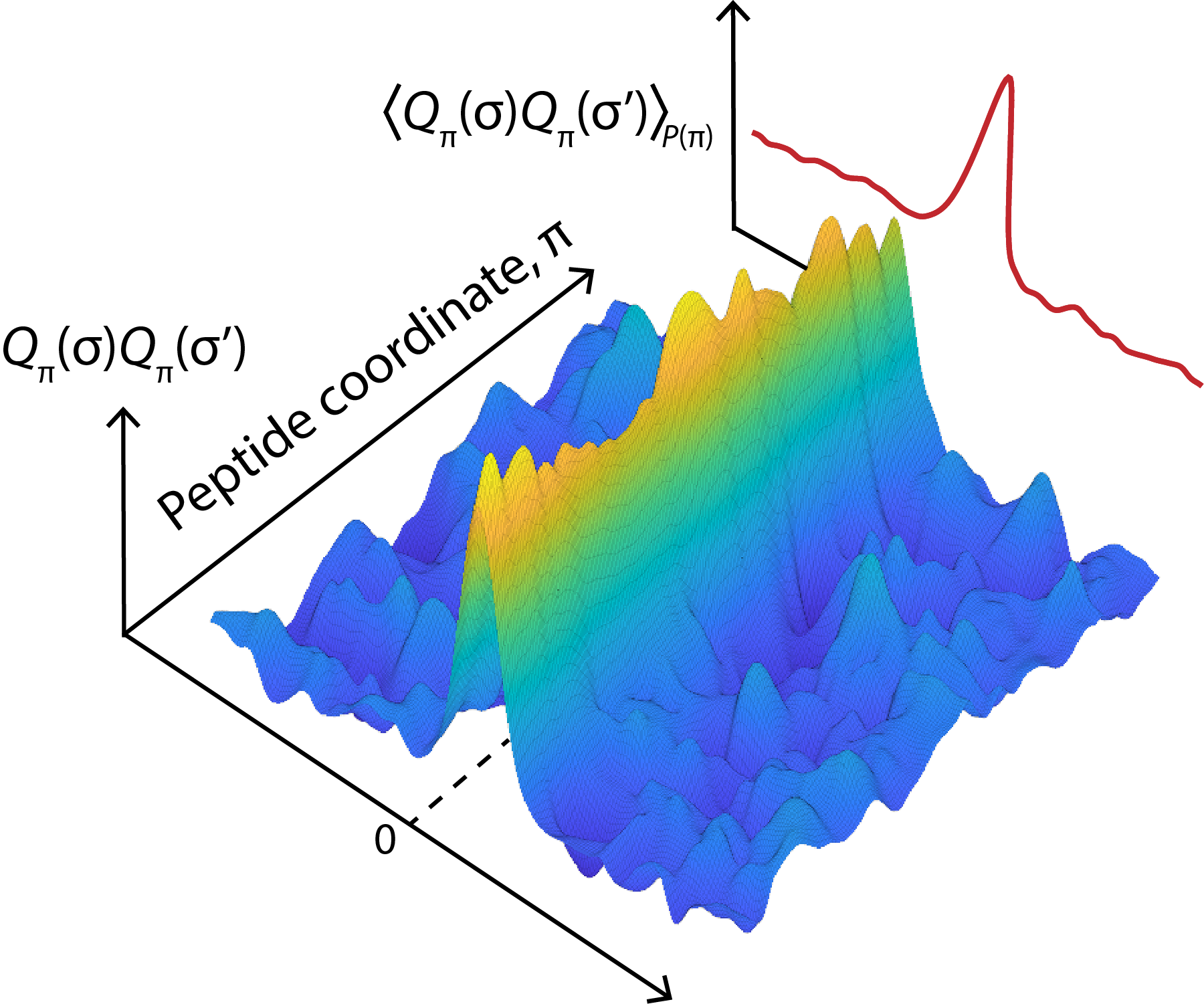
Cellular immunity relies on the specific recognition of target molecules by T cells, mediated by the binding of the T cell receptor (TCR) to specific peptide-major histocompatibility complexes (pMHCs). Given the pivotal role of T cells in the adaptive immune system, the ability to predict the specificity of a TCR in silico from its sequence has numerous potential applications in disease diagnosis, surveillance, and treatment. Recent experimental advances and the creation of databases cataloging known TCR-pMHC pairings have propelled significant research efforts aimed at predicting TCR specificity using machine learning. This approach is based on the premise that there are learnable, generalizable biophysical rules governing TCR-pMHC interactions.
I am particularly interested in exploring: What underlying biophysical rules that govern the specificity of TCR-pMHC interactions? How can machine learning models capture these biophysical rules to improve prediction accuracy? What role do sequence and structural features play in defining TCR specificity, and how can these insights be applied to therapeutic design?
- Data-driven discovery of biophysical T cell receptor co-specificity rules. PRX Life (2025)
Pyo, A. G. T., Nagano, Y., Milighetti, M., Henderson, J., Callan Jr., C. G., Chain, B., Wingreen, N. S., & Tiffeau-Mayer, A. - Contrastive learning of T cell receptor representations. Cell Systems (2025)
Nagano, Y., Pyo, A. G. T., Milighetti, M., Henderson, J., Shawe-Taylor, J., Chain, B., & Tiffeau-Mayer, A.
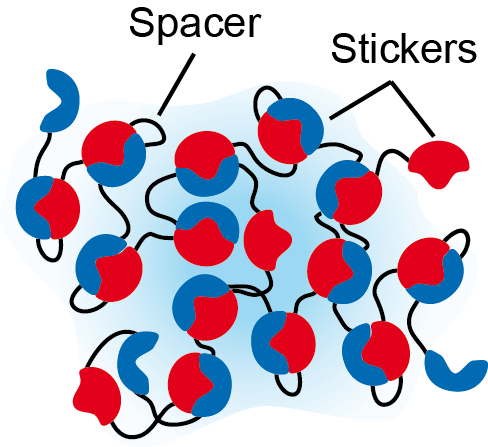
Living cells must exert both spatial and temporal control of their internal components to successfully carry out desired tasks. One ubiquitous mode of control is compartmentalization of internal components through the formation of biomolecular condensates. These condensates are understood to form through liquid-liquid phase separation of biopolymers, such as proteins and nucleic acids, which form a scaffold for other molecules to colocalize in the condensates. Biomolecular condensates possess liquid characteristics such as fusion, flow, and continuous material exchange between dense and dilute phases, leading to functional advantages such as reversible response to environmental conditions, rapid internal mixing of components, and ready recruitment of reactants necessary for biochemistry or signaling. Ultimately, these macroscopic properties of the condensates are encoded in the microscopic features of the constituent biopolymers. Is there a systematic way to bridge between macro and microscales?
- Proximity to criticality predicts surface properties of biomolecular condensates. PNAS (2023)
Pyo, A. G. T., Zhang, Y., & Wingreen, N. S. - Surface tension and super-stoichiometric surface enrichment in two-component biomolecular condensates. iScience (2022)
Pyo*, A. G. T., Zhang*, Y., & Wingreen, N. S.
Preprints
- Membrane wetting by biomolecular condensates is facilitated by mobile tethers. (2024)
GrandPre, T., Pyo, A. G. T., & Wingreen, N. S.
Publications
- Evolutionary benefits of fitness-dependent mutation rates. PRL (2025)
Pyo, A. G. T., Yu, Q., Merkenschlager, J., & Wingreen, N. S. - Data-driven discovery of biophysical T cell receptor co-specificity rules. PRX Life (2025)
Pyo, A. G. T., Nagano, Y., Milighetti, M., Henderson, J., Callan Jr., C. G., Chain, B., Wingreen, N. S., & Tiffeau-Mayer, A. - Regulated somatic hypermutation enhances antibody affinity maturation. Nature (2025)
Merkenschlager*, J., Pyo*, A. G. T., Silva Santos, G. S., Schaefer-Babajew, D., Cipolla, M., Hartweger, H., Gitlin, A. D., Wingreen, N. S., & Nussenzweig, M. C. - Contrastive learning of T cell receptor representations. Cell Systems (2025)
Nagano, Y., Pyo, A. G. T., Milighetti, M., Henderson, J., Shawe-Taylor, J., Chain, B., & Tiffeau-Mayer, A. - The exchange dynamics of biomolecular condensates. eLife (2024)
Zhang, Y., Pyo, A. G. T., Jiang, Y., Brangwynne, C. P., Stone, H. A., & Wingreen, N. S. - Impact of linker length on biomolecular condensate formation. PRX Life (2023)
GrandPre, T., Zhang, Y., Pyo, A. G. T., Weiner, B., Li, J. L., Jonikas, M. C., & Wingreen, N. S. - Proximity to criticality predicts surface properties of biomolecular condensates. PNAS (2023)
Pyo, A. G. T., Zhang, Y., & Wingreen, N. S. - Surface tension and super-stoichiometric surface enrichment in two-component biomolecular condensates. iScience (2022)
Pyo*, A. G. T., Zhang*, Y., & Wingreen, N. S. - Motif-pattern dependence of biomolecular phase separation driven by specific interactions. PLOS Computational Biology (2021)
Weiner, B. G., Pyo, A. G. T., Meir, Y., & Wingreen, N. S. - Memory effects in single-molecule force spectroscopy measurements of biomolecular folding. PCCP (2019)
Pyo, A. G. T., & Woodside, M. T. - Measuring the average shape of transition paths during the folding of a single biological molecule. PNAS (2019)
Hoffer, N. Q., Neupane, K., Pyo, A. G. T., & Woodside, M. T. - Transition-path properties for folding reactions in the limit of small barriers. Journal of Chemical Physics (2018)
Pyo, A. G. T., Hoffer, N. Q., Neupane, K., & Woodside, M. T. - Probing Position-Dependent Diffusion in Folding Reactions Using Single-Molecule Force Spectroscopy. Biophysical Journal (2018)
Foster, D. A. N., Petrosyan, R., Pyo, A. G. T., Hoffmann, A., Wang, F., & Woodside, M. T.